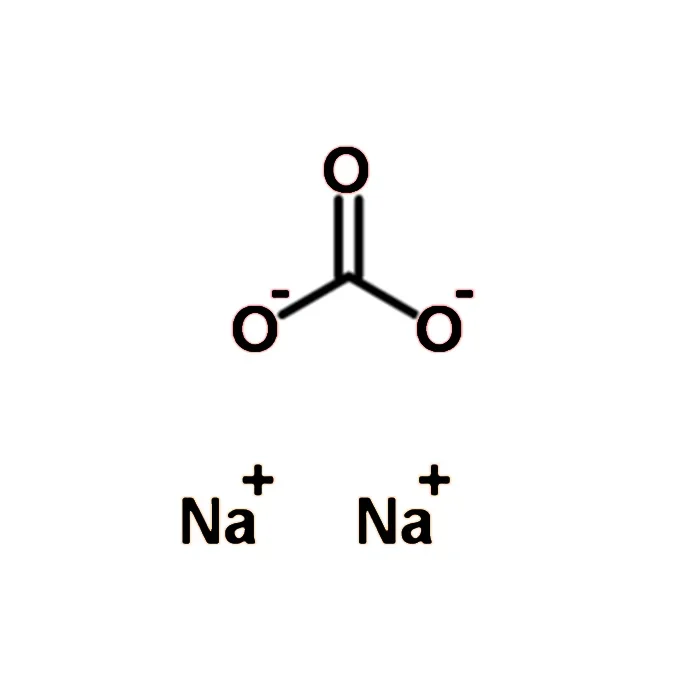
Sodium carbonate, commonly known as soda ash or washing soda, is a widely used chemical compound with the chemical formula Na2CO3. In its sterile powder form, it is particularly important in various industrial, medical, and scientific applications due to its unique physical properties. In this blog post, SACH BIOTECH will provide you with the physical properties of sodium carbonate sterile powder, its production methods, and its uses in different sectors.
Introduction to Sodium Carbonate Sterile Powder
Sodium carbonate is a white crystalline solid that is highly soluble in water. The sterile powder form is especially prepared to ensure that it is free from any bacterial, fungal, or viral contaminants, making it suitable for use in sensitive environments such as pharmaceuticals and medical procedures.
Physical Properties of Sodium Carbonate Sterile Powder
1. Appearance: Sodium carbonate sterile powder is a white, odorless, and fine crystalline powder.
2. Solubility: It is readily soluble in water, with a solubility of about 22 g per 100 mL of water at 20°C. This property makes it an excellent cleaning agent and a key component in various chemical reactions.
3. Density: The density of anhydrous sodium carbonate is approximately 2.53 g/cm³.
4. Melting Point: The melting point of sodium carbonate is around 851°C (1,557°F), which indicates its stability at high temperatures.
5. Boiling Point: When heated, sodium carbonate does not boil but decomposes at approximately 1,600°C (2,912°F).
6. Hygroscopicity: Sodium carbonate is slightly hygroscopic, meaning it can absorb moisture from the air, which is why it is often stored in airtight containers.
7. pH: In aqueous solution, sodium carbonate is strongly alkaline, with a pH typically above 11.
8. Reactivity: Sodium carbonate is a basic salt and can react with acids to produce carbon dioxide gas, water, and the corresponding salt.
Production Methods of Sodium Carbonate Sterile Powder
1. Solvay Process: Also known as the ammonia-soda process, this is the most common industrial method for producing sodium carbonate. It involves the reaction of sodium chloride (salt), ammonia, and carbon dioxide.
2. Henderson Process: This method is similar to the Solvay process but uses different starting materials and is less common.
3. Biological Production: Some bacteria and algae can produce sodium carbonate as a byproduct of their metabolism, which can then be harvested and processed.
4. Sterilization: Regardless of the production method, the final step to obtain sterile powder involves sterilization techniques such as autoclaving or gamma irradiation to ensure the product is free from contaminants.
Applications of Sodium Carbonate Sterile Powder
1. Pharmaceuticals: Used as an excipient in the pharmaceutical industry for tablet and capsule formulations.
2. Cleaning Agents: Its alkaline nature makes it a common ingredient in detergents and cleaning products.
3. Glass Manufacturing: Sodium carbonate is a key ingredient in the production of glass, as it lowers the melting point of silica.
4. Food Industry: Used as a leavening agent in the baking industry and as a pH regulator in various food products.
5. Water Treatment: It is used to adjust the pH in water treatment processes and to soften water by precipitating calcium and magnesium ions.
6. Scientific Research: Sterile sodium carbonate is essential in laboratories for various chemical and biological experiments.
7. Textile Industry: Utilized in the processing of textiles to remove impurities and to assist in dyeing processes.
8. Photography: Sodium carbonate plays a role in the development of photographic film.
Safety and Handling of Sodium Carbonate Sterile Powder
1. Personal Protective Equipment (PPE): When handling sodium carbonate sterile powder, it is advisable to wear gloves, safety goggles, and a lab coat or apron to prevent skin and eye irritation.
2. Storage: Store in a cool, dry place in a sealed container to prevent moisture absorption and contamination.
3. Disposal: Dispose of sodium carbonate in accordance with local regulations, as it can be harmful to aquatic life in high concentrations.
4. First Aid: In case of accidental ingestion, drink plenty of water and seek medical attention. For skin or eye contact, rinse with water and seek medical advice if irritation persists.
Conclusion
Sodium carbonate sterile powder, with its unique physical properties and broad applications, is an essential compound in various industries. Understanding its characteristics, production methods, and applications can help in the safe and effective use of this versatile substance. As with any chemical, it is crucial to handle sodium carbonate sterile powder with care and to follow safety guidelines to ensure the well-being of both individuals and the environment.
https://www.hzsqchem.com/Physical-properties-of-sodium-carbonate-sterile-powder.html
SACH
sales@hzsqchem.com